Abstract
Background We previously performed an integrative genomic analysis for 47 chronic phase (CP)-CML patients at diagnosis who were specifically selected based on optimal or very poor outcome to tyrosine kinase inhibitors (TKIs). Patients who subsequently progressed to blast phase (BP) had a significantly higher frequency of cancer-related gene mutations at diagnosis compared with patients who achieved an optimal response (54% versus 16%). A novel mutational subtype associated with the formation of the Philadelphia (Ph) chromosome, termed Ph-associated rearrangements, were also described and included novel fusions, deletions and inversions adjacent to the BCR::ABL1 junction. These occurred at a higher frequency of patients who progressed to BP compared to those with an optimal response (33% versus 11%). Consequently, we sought to investigate the true incidence and effect of cancer-gene mutations and Ph-associated rearrangements (collectively termed additional mutational events, AMEs) detected at diagnosis and whether outcomes differ based on the TKI initiated.
Aim To assess the impact on outcome and molecular response of AMEs detected at diagnosis for patients consecutively treated with imatinib compared to patients treated with second-generation TKIs (2G-TKI).
Methods A recently validated hybridization capture sequencing method targeting genes involved in both myeloid and lymphoid malignancies was applied to available diagnostic RNA of consecutive clinical trial patients treated with either frontline imatinib or 2G-TKIs. The 200 imatinib-treated patients (TIDEL II; CML9) were commenced on 600mg daily of imatinib and managed with a highly proactive treatment intervention strategy for suboptimal response, which was either imatinib dose increase or switch to nilotinib. The 2G-TKI patients were enrolled in either DIRECT;CML12 (n=72), where patients commenced dasatinib 100mg daily before dose modification, or either ENESTxtnd or PINNACLE;CML11 (n=44) where patients commenced nilotinib 300mg twice daily. Variants in cancer genes with variant allele frequencies ≥5%, including single nucleotide variants, RNA splice altering variants, small insertions/deletions, gene fusions and focal gene deletions were assessed for pathogenicity using strict criteria. Ph-associated rearrangements were identified. Clinically relevant variants were confirmed as somatic and validated. Kaplan-Meier and cumulative incidence analyses were performed to assess the effect of AMEs on failure-free survival and key molecular outcomes by 36 months. Failure events were as defined by the European LeukemiaNet 2020.
Results The incidence of AMEs for imatinib-treated patients was 31% (61/200): 33 (16%) cancer gene mutations and 36 (18%) Ph-associated rearrangements; 8 patients had both subtypes. In the 2G-TKI group, 41 AMEs were identified in 29% (34/116) of patients: 20 (17%) cancer gene mutations and 16 (14%) Ph-associated rearrangements; 2 patients had both subtypes. In total, 14 cancer genes were mutated. Consistent with other studies, ASXL1 mutations were most frequently detected: 18/200 imatinib patients (9%) and 9/116 2G-TKI patients (8%).
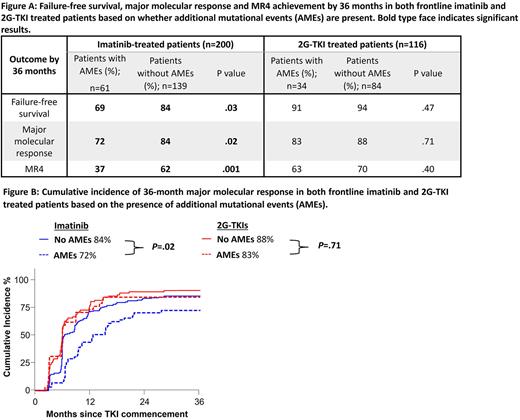
Using 36-month outcomes as comparison, imatinib-treated patients with AMEs had inferior failure-free survival (84% versus 69%, P=.03), major molecular response (84% versus 72%, P=.02) and MR4 (62% versus 37%, P=.001). In contrast, outcomes in the 2G-TKI cohort were similar irrespective of the presence of AMEs, with regards to failure-free survival (94% versus 91%, P=.47) and achievement of major molecular response (88% versus 83%, P=.71). Interestingly, achievement of MR4 was delayed for patients with AMEs compared to those without AMEs at diagnosis: 51% versus 69%, P=.02 by 24 months; and 63% versus 70%, P=.40 by 36 months. No differences were observed for overall survival or transformation-free survival in either the frontline imatinib or 2G-TKI cohorts.
Conclusion These results indicate that while the presence of AMEs do predict for inferior outcomes and molecular responses when patients are treated with frontline imatinib, the more potent 2G-TKIs may overcome the negative impact of the AMEs. The effect for imatinib treated patients was evident despite a starting dose of 600mg and more stringent treatment intervention than currently recommended. This supports a strategy of mutation analysis at diagnosis of CP-CML for optimal TKI selection.
Disclosures
Shanmuganathan:Novartis: Honoraria; Amgen: Other: Meeting sponsorship. Yeung:Amgen: Honoraria; Pfizer: Honoraria; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Honoraria, Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees. Ross:BMS: Honoraria; Celgene: Research Funding; Keros: Consultancy; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees. Hughes:Enliven: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; BMS: Consultancy, Research Funding. Branford:Cepheid: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Qiagen: Honoraria; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal